Eko Health Launches CORE 500™ Digital Stethoscope with Heart Disease Detection AI, 3-Lead ECG, and Advanced Audio Capabilities
Next-generation FDA-cleared medical device brings non-invasive heart and lung disease detection to healthcare professionals at the point of care.
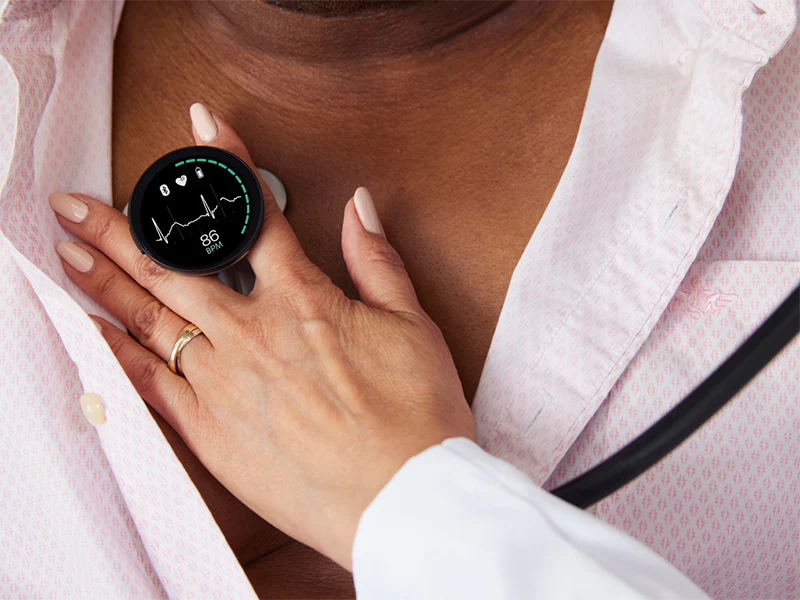
Emeryville, California - June 27, 2023 - Eko Health, a leading innovator in digital health technology for heart and lung disease detection, today announced the launch of its next-generation digital stethoscope, CORE 500™. The CORE 500™ Digital Stethoscope represents over 10 years of pioneering the industry of digital auscultation (listening to heart, lung, and body sounds). Engineered to enhance diagnostic accuracy and early disease detection, the CORE 500™ combines artificial intelligence (AI) software, high-fidelity audio experience, full-color display, and 3-lead electrocardiogram (ECG) to deliver an essential patient assessment tool for healthcare professionals (HCPs).
“A few years ago, we set out to reimagine the most ubiquitous medical tool. We’ve spent countless hours designing, testing, and refining to produce a flagship product that we’re very proud of and has high clinical value — the CORE 500™ is the result,” said Connor Landgraf, Co-founder & CEO of Eko Health. “The CORE 500™ represents a major leap forward in digital stethoscope technology. When paired with our powerful AI algorithms, it can help detect leading indicators of heart disease in seconds, greatly assisting healthcare professionals in identifying various cardiac conditions with increased accuracy and confidence. This innovative feature aims to save time during routine exams and improve patient outcomes.”
The CORE 500™ is the first stethoscope to provide HCPs with accurate, instantaneous patient auscultation data on a full-color display, including heart rate and ECG tracing. When supported by Eko Health’s FDA-cleared AI products, the stethoscope flags abnormalities, including AFib, bradycardia, and tachycardia, in seconds. The CORE 500™ is equipped with up to 40x sound amplification, active noise cancellation, three audio filter modes, and features Eko's newest audio innovation, TrueSound™. With in-ear speaker technology, TrueSound™ offers optimal sound filtering and reduced background noise to provide the most precise sound during every patient exam.
With a conventional stethoscope's familiar design and intuitive workflow, CORE 500™ is designed for use across clinical specialties, including structural heart, primary care, urgent care, emergency medicine, home care, and virtual care. Built for dependability, the CORE 500™ is made from lightweight shatter-, splash-, and scratch-resistant material. Its fast-charging lithium-ion battery is optimized for multiple shifts, lasting up to 60 hours of regular clinical use on a single charge.
“As cardiovascular disease rises with our aging population, noninvasive tools like the CORE 500™ turn a patient interaction into a potential disease screening,” said Paul Friedman, M.D., Chair of Cardiovascular Medicine at Mayo Clinic in Rochester, Minnesota. The Mayo Foundation for Medical Education and Research has a financial interest in Eko Health. "Supported AI diagnostics software, advanced audio technology, and 3-lead ECG make this a useful tool for clinicians. The live ECG tracing on the display will help identify patients with previously unknown arrhythmias at the point of care."
CORE 500™ will be compatible with Eko Health’s SENSORA™ Cardiac Disease Detection Platform. SENSORA™ is the first advanced applied machine learning software that more than doubles the detection sensitivity of cardiac murmurs indicative of structural heart disease, giving health systems the power to optimize downstream care and improve outcomes. The CORE 500™ also seamlessly integrates with Eko Live Stream for synchronous listening that allows virtual teams to provide patient care with enhanced capability to record, analyze, and share auscultation data in real-time from anywhere. To learn more about the availability of CORE 500™ for your health system, please contact Eko Health at ekohealth.com/enterprise.
The CORE 500™, which recently received FDA 510(k) clearance, is available exclusively at ekohealth.com.
About Eko Health
Eko Health is a leading digital health company advancing how healthcare professionals detect and monitor heart and lung disease with its portfolio of digital stethoscopes, patient and provider software, and AI-powered analysis. Its FDA-cleared platform is used by hundreds of thousands of healthcare professionals worldwide, allowing them to detect earlier and with higher accuracy, diagnose with more confidence, manage treatment effectively, and ultimately give their patients the best care possible. Eko Health is headquartered in Emeryville, California, with over $125 million in funding from Highland Capital Partners, Questa Capital, Artis Ventures, DigiTx Partners, NTTVC, Morningside Technology Ventures Limited, Mayo Clinic, Sutter Health, and others. For more information visit www.ekohealth.com.
MKT-0002019 v.2.0
